Nationwide Recall of Robitussin Honey CF Max Day Adult and Robitussin Honey CF Max Nighttime Adult Products
 It’s flu season and you might have a bottle of Robitussin Cold and Flu cough syrup in your cabinet.
It’s flu season and you might have a bottle of Robitussin Cold and Flu cough syrup in your cabinet.
You might want to check the label because this week, the Company that makes Robitussin products, Haleon, is recalling Honey CF Max Day Adult and Robitussin Honey CF Max Nighttime Adult cough syrup due to microbial contamination.
Haleon is voluntarily recalling eight lots of Robitussin Honey CF Max Day Adult and Robitussin Honey CF Max Nighttime Adult to the consumer level.
In immunocompromised individuals, the use of the affected product could potentially result in severe or life-threatening adverse events such as fungemia (fuhng·geh·mee·uh)
or disseminated fungal infection.
In otherwise healthy consumers, the population most likely to use the product, life-threatening infections are not likely to occur.
However, the occurrence of an infection that may necessitate medical intervention cannot be completely ruled out.
To date, Haleon has not received any reports of adverse events related to this recall.
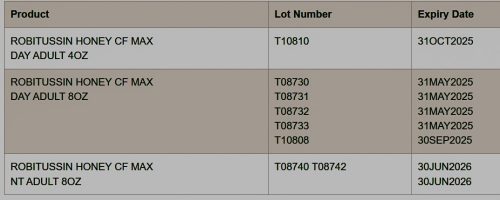
Robitussin Honey CF Max Day and Nighttime are cough syrups indicated for the temporary relief of symptoms occurring with cold or flu, hay fever, or other respiratory allergies. This recall covers only the following lots:
Haleon is notifying its distributors and customers directly and has provided them with instructions for the return of all recalled products.
Consumers who have purchased the product listed should stop consumption immediately.
Please call our Consumer Relations team at +1-800-245-1040 (Monday through Friday 8 AM to 6 PM Eastern Time) or reach out via email to [email protected].
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
Complete and submit the report Online: www.fda.gov/medwatch/report.htm
Regular Mail or Fax: Download the form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
